<< previous |
I’ll pick up from the previous post on design covalent inhibitors of SARS-CoV-2 main protease (structure and chart numbering follows from there). As noted previously, I really think that you need to exploit conserved structural features, such as the catalytic residues and the oxyanion hole, if you’re genuinely concerned about resistance and I do consider it a serious error to make a virtue out of non-covalency. As in the previous post, I've linked designs to the original Covid Moonshot submissions whenever possible.
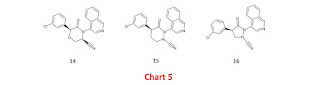
I’ll kick the post off with 14 (Chart 5) which replaces a methylene in the lactam ring of 10 (Chart 4 in previous post) with oxygen. This structural transformation results in 0.8 log unit reduction in lipophilicity (at least according to the algorithm used for the Covid Moonshot) and might also simplify the synthesis.
Designs 15 and 16 (also in Chart 5) link the nitrile warhead from nitrogen rather than carbon and this structural transformation eliminates a chiral centre in each of 10 and 11 (Chart 4 in previous post) and may be beneficial for affinity (see discussion around 8 and 9 in Chart 3 of the previous post). In substituted hydrazine derivatives, the nitrogen lone pairs (or the π-systems which the nitrogens are in) tend to avoid each other and so I’d expect nitrile warheads of 15 and 16 to adopt axial orientations. I’d anticipate that the nitrile warhead will be directed toward the catalytic cysteine for 15 but away from the catalytic cysteine for 16 and I favor the former for this for this reason. It's also worth mentioning that even if the nitrile is directed away from the catalytic cysteine it may occupy the oxyanion hole.
I’ll finish with couple of designs based on aromatic sulfur that are shown in Chart 6. Design 17 was originally submitted by Vladas Oleinikovas although I’ll also link my resubmission of this design because the notes include a detailed discussion of a design rationale along with a proposed binding mode. My view is that the catalytic cysteine could get within striking distance of the ring sulfur (which can function as a chalcogen-bond donor and potentially even an electrophile). Although 2,1-benzothiazole is not obviously electrophilic, it’s worth noting that acetylene linked by saturated carbon can replace the nitrile as an electrophilic warhead (this isosteric replacement leads to irreversible inhibition as discussed in this article). I’ve also included 18 which replaces 2,1-benzothiazole with (what I’d assume is) a more electrophilic heterocycle. I would anticipate that any covalent inhibition by these compounds will be irreversible.

No comments:
Post a Comment